The production process of API is complex, with flammability, explosiveness, and toxicity. The hazards mainly include the following aspects:
1. Material aspect
The materials used in production are mostly flammable and explosive substances. Once the materials leak or are of poor quality, they can cause explosion accidents.
2. Equipment design and manufacturing issues
3. Unreasonable layout design issues
Unreasonable design of functional areas distribution, explosion-proof area setting, HVAC design, equipment layout, etc. in the factory building can all lead to the occurrence of explosion accidents during the production process.
4. Production operations do not comply with standards
The operation personnel did not operate according to the operating procedures, and did not timely detect changes in equipment process parameters during equipment operation, such as excessive catalyst dosage or too fast feeding speed; Blindly pursuing production, equipment over pressure, overload operation, etc.
Canaan Technology understands and comes into contact with common problems of raw material manufacturing enterprises in the design and implementation of raw material engineering projects, and combines itself to make technological improvements and optimization, which are applied to every raw material engineering project we serve.
Canaan Technology’s safety measures for raw material engineering projects, starting from the design, are mainly reflected in the following aspects:
1. Ensure security from process design
The production process of active pharmaceutical ingredients has high requirements for pipeline materials, pipe diameters, connection methods, valve fittings types, valve setting points, etc. When designing, full consideration should be given to the safety accessory types, setting parameters, settings, and pipeline systems (process materials and waste water and exhaust gas pipeline systems) of valve fittings.
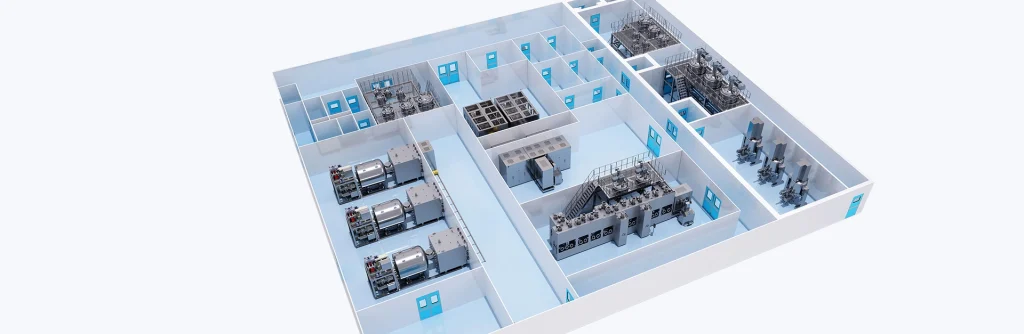
2. Ensure safety from the rationality of factory process layout
In process layout design, optimize the configuration of equipment layout, functional area distribution, explosion-proof area distribution, pressure relief direction area, operation and maintenance space, and evacuation routes.
3. Ensure safety in equipment design and selection
In the process of equipment design, selection, and manufacturing, attention should be paid to monitoring the working and manufacturing parameters of the equipment, especially the selection and configuration optimization of equipment and key components.
4. The project adopts three-dimensional design to ensure safety
The project engineering uses visualized 3D design and simulated installation to calibrate and optimize the pipeline system and process layout, and verify the safety and reliability of the project.
5. Optimize electrical automation control design to ensure safety
A reasonable and optimal self-control design can better ensure project safety, including project process alarms, self-control systems, online monitoring systems for waste water and exhaust gas content, and CIP/SIP self-control systems.
6. Ensure safety from the perspective of environmental regulations and requirements
From a safety perspective, catalytic oxidation and other methods are used to treat exhaust gas to meet environmental requirements.
Canaan Technology: Focus on Health Value
As a pharmaceutical equipment enterprise that shoulders social responsibility, Canaan Technology adheres to the concept of “focusing on safety and quality from the design source”, providing safe and reliable measures for customers’ raw material engineering in various aspects such as raw material supply, process control, quality control, installation and commissioning, pipeline construction, and acceptance, and safeguarding the production safety of every customer.




Manufacturing pharmaceutical products should always be taken seriously. That is, every process must follow the strictest and highest standards. This is the very reason why manufacturers prefer hiring an EPC contractor. Contractors working under EPC contracts will ensure the outcomes are of the best quality no matter what happens, focusing on the construction of the […]

Explore the importance of EPC contracts in pharmaceutical manufacturing. Learn how EPC works, its benefits, and why choosing an EPC contractor can guarantee project success with Canaan’s industry-leading equipment.
Discover how SCADA and PLC improve automation in the pharmaceutical industry. Learn their roles, benefits, and how Canaan’s advanced technology enhances efficiency and safety.