Introduction
In the context of the continuous development of the global pharmaceutical industry, drug safety and production efficiency have become the core goals pursued by various pharmaceutical companies. In recent years, with the gradual updating and improvement of the appendix of the “Good Manufacturing Practice for Drugs” (2010 version GMP) regulations, the country’s supervision and requirements for the pharmaceutical industry have become increasingly strict.
In the face of these challenges, Canaan Technology is no longer satisfied with assisting customers in providing verification documents. It can now provide full process verification from device installation to performance qualification (PQ), with comprehensive service upgrades.
Verify Service Cases
Taking a pharmaceutical company in Tianjin as an example, the customer has newly purchased a Coater with Perforated Drum and a Post Bin Blender, and Canaan Technology is responsible for the design, production, installation, commissioning, testing, and verification work.
After the equipment arrives at the workshop, both parties jointly determine the verification plan and conduct equipment verification.


▲ Equipment Display Diagram
Canaan Verification Service Process

▲ Verification Service Flow Chart
According to the flowchart, Canaan Technology provides the following verification services to customers:
Phase 1: User Requirements URS
Canaan Technology has had in-depth communication with customers to ensure that the equipment validation plan, execution, reporting, rectification, and deviation handling can meet GMP requirements. The equipment validation engineers, based on their own experience, complete the validation work on time and with quality within the planned time, and promptly follow up and handle deviations or changes.
Phase 2: System Risk Assessment RA
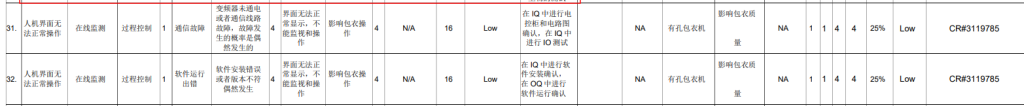
▲ Project Information Diagram
Phase 3: Confirmation/Verification Activities
The validation life-cycle of the equipment mainly adopts the equipment validation process proposed in the 2023 GMP-API “Facility and Equipment Confirmation”, which covers the following stages:
Verification Results

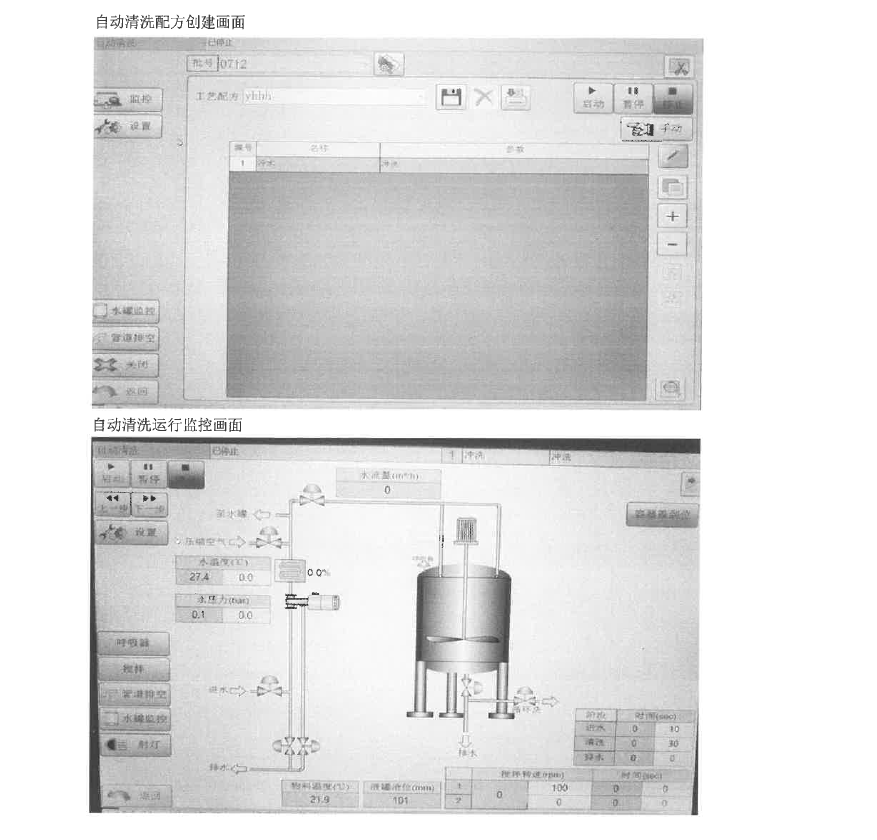
▲ Verify Document Diagram
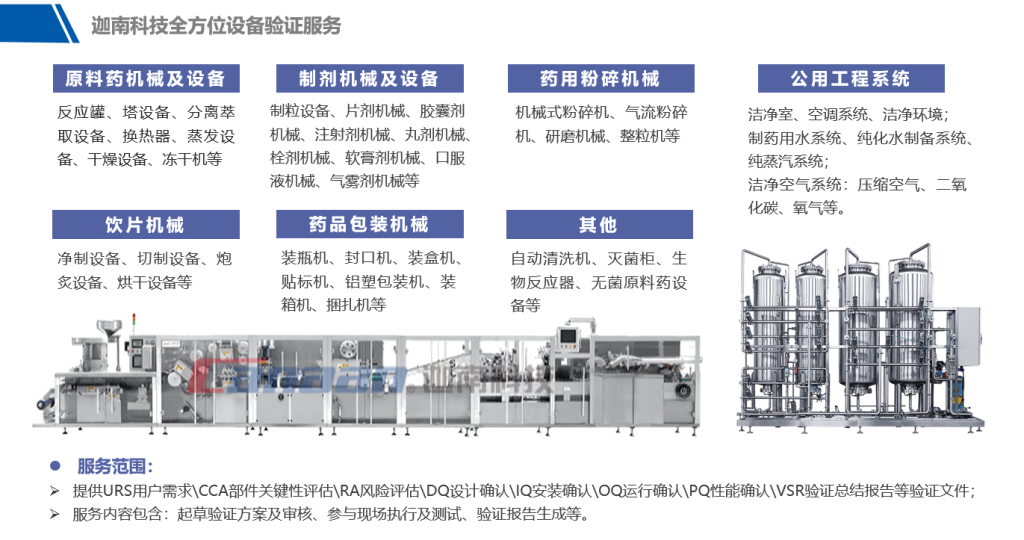
Canaan Technology: A Reliable Verification Service Provider
Canaan Technology’s regulatory experts have extensive experience and have completed equipment validation and CSV validation for numerous pharmaceutical clients in China. They can provide efficient compliance consulting and validation services to enhance the performance and reliability of pharmaceutical equipment, ensuring its compliance and efficient operation in pharmaceutical environments.




Introduction: Aseptic Isolators in High-Containment Scenarios The demand for aseptic isolators is increasing due to the growing need for oncology therapies and strict regulatory standards like ISO 14644 and EU GMP. The pharmaceutical industry is heavily investing in research and development to create new treatments. Market dynamics, including demand trends, regulatory challenges, and technological advancements, […]

Maintaining impeccable cleanliness is the foundation of pharmaceutical manufacturing, and bin washing stations play a pivotal role in achieving this. These systems are designed to efficiently clean and sanitize bins, containers, and other equipment used in handling sensitive materials. But how do they work? Let’s break down the process step by step to give you […]

Key Takeaways What Is a Containment Isolator? Unveiling the Safety Shield in Pharmaceutical Operations Containment isolators, like the soloADC™ Disposable Containment System, are designed to ensure safety when handling hazardous substances. They are enclosed workspaces that create a physical barrier between the operator and the hazardous materials. This barrier is crucial for protecting operators, preventing […]