In the development of innovative drugs, process optimization of generic drugs and scientific research scenarios in universities, traditional Fluid-bed equipment faces three core challenges:
1. Insufficient batch adaptability: Conventional equipment processing capacity starts at kilograms, which cannot meet the needs of 100g-level micro-experiments, resulting in raw material waste and a surge in R&D costs;
2. Single function and space limitation: Drying, granulation, coating and other processes require multiple equipment to cooperate, laboratory space utilization is low and operation is complicated;
3. Data traceability and compliance pressure: Traditional equipment lacks refined parameter control and data recording functions, and it is difficult to meet GMP requirements for process verification and traceability.
In response to the above points, Canaan Technology launched the FZM Series Fluid-bed Granulator, with “miniaturization, modularization, and intelligence” as the core, redefining laboratory-level solid dosage development tools.
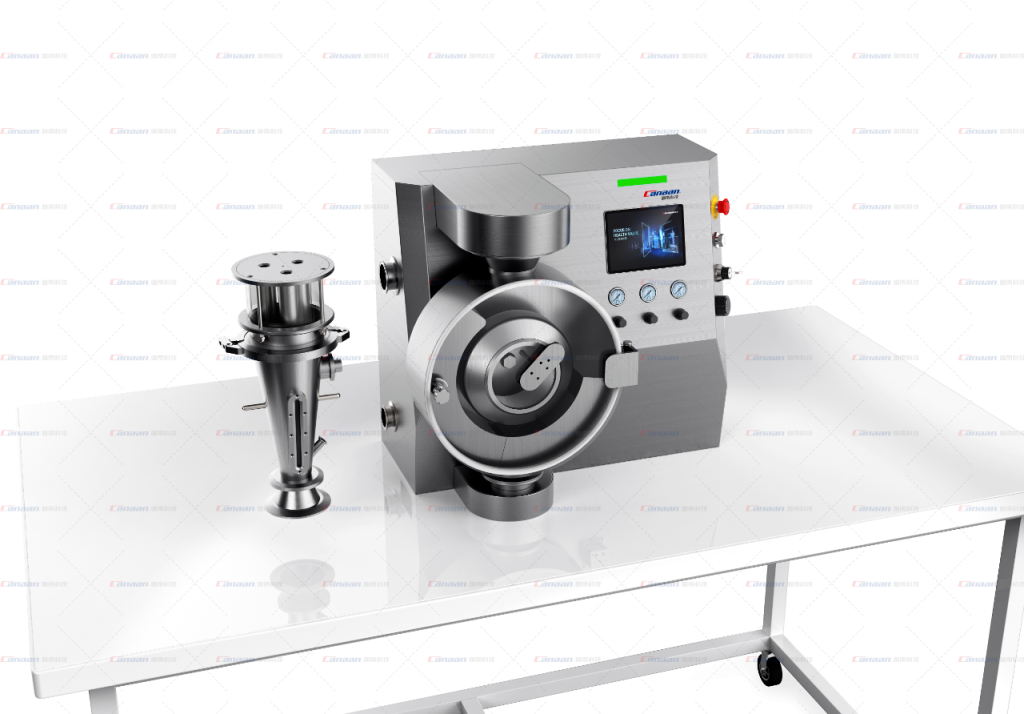
1. Applicable Scenarios:
• Process exploration of high-value APIs (such as ADC drugs and peptides);
• Prescription optimization and process verification in the consistency evaluation of generic drugs;
• Innovative dosage form development in universities and research institutes (such as sustained-release pellets and orodispersible films).
2. Processing Batch: 100g-200g (micro-scale experimental requirements);
3. Functional Coverage: drying, granulation, pellet/powder/tablet coating;
4. Space Design: The size of the whole machine is adapted to the laboratory table, supporting “plug and play” deployment.
• Integrated architecture: Integrates air blowing, heating, slurry addition and control systems to reduce reliance on external equipment;
• Quick switching module: The function of the Fluid-bed and coating pan can be switched through a clamp connection, and the process switching can be completed within 5 minutes (traditional equipment takes several hours);
• Convenience of mobility: The weight of the whole machine is optimized to be portable by two people, supporting flexible deployment across laboratories.
•Full process coverage: a single device supports key processes such as drying, granulation, and coating;
•Micro-process adaptation: fluidized air duct and atomization system designed specifically for small batches, ensuring process stability as low as 100g batches.
• Free parameter configuration: multiple sets of parameters can be set by yourself, covering variables such as temperature and injection rate;
• Real-time monitoring and traceability: built-in sensors can realize millisecond-level acquisition of key data (such as bed temperature and pressure gradient), in compliance with FDA 21 CFR Part 11 electronic record regulations;
• Cloud collaboration: optional data export function, directly connected to the LIMS system, to accelerate the transfer of knowledge from R&D to production.

• Cost optimization: micro-scale processing reduces the loss of high-value raw materials and reduces the cost of a single experiment by 70%;
• Efficiency breakthrough: module switching and parameter preset functions shorten the process development cycle;
• Compliance guarantee: fully enclosed design + SUS316L material, meeting GMP requirements for cross-contamination prevention and control and cleaning verification.
With the popularization of personalized drug development and continuous manufacturing technology, laboratory equipment is accelerating its iteration towards “micro, intelligent, and compliant“. In the future, Canaan Technology will continue to deepen the process optimization algorithm enabled by AI and promote the evolution of desktop equipment from “experimental tools” to “R&D brains”.




Manufacturing pharmaceutical products should always be taken seriously. That is, every process must follow the strictest and highest standards. This is the very reason why manufacturers prefer hiring an EPC contractor. Contractors working under EPC contracts will ensure the outcomes are of the best quality no matter what happens, focusing on the construction of the […]

Explore the importance of EPC contracts in pharmaceutical manufacturing. Learn how EPC works, its benefits, and why choosing an EPC contractor can guarantee project success with Canaan’s industry-leading equipment.
Discover how SCADA and PLC improve automation in the pharmaceutical industry. Learn their roles, benefits, and how Canaan’s advanced technology enhances efficiency and safety.